Introduction
The recent approvals of several effective targeted therapies have shifted treatment paradigms for chronic lymphocytic leukemia (CLL). The rapid pace of approvals and expanded indications may challenge oncology healthcare providers (HCPs) to make optimal treatment decisions.
We developed an online treatment decision support tool designed to provide HCPs with case-specific treatment recommendations from five CLL experts; this tool has been regularly updated since 2017. In this analysis, we examined the CLL treatment patterns of HCPs and experts from three tool iterations-spanning early 2017 to August 2020-and evaluated comparative trends over time.
Methods
For each version of the CLL tool, five experts provided treatment recommendations for hundreds of different case scenarios in the newly diagnosed and relapsed/refractory CLL settings. These unique case scenarios were defined by patient and disease factors that the experts considered critical to making treatment decisions, including patient age and fitness, cytogenetic abnormalities, and previous treatment. To use the tool, HCPs entered patient information and their intended treatment plan; expert recommendations for that specific patient scenario were then provided, followed by a survey to determine whether the recommendations impacted the HCP's intended treatment.
This analysis compared the intended treatment plans for cases entered into the tool by HCPs with expert recommendations in three versions of the tool (March 2017, October 2018, and October 2019). HCP responses to the post-recommendation survey also were assessed.
Results
HCPs sought recommendations for 543 cases from March to July 2017 for the 2017 CLL tool, for 656 cases from October 2018 to July 2019 for the 2018 tool, and for 1015 cases from October 2019 to August 2020 for the 2019 tool. HCPs were generally less experienced with CLL; for example, in the 2019 tool, 48% of HCPs who sought treatment recommendations treated ≤ 10 patients with CLL per year.
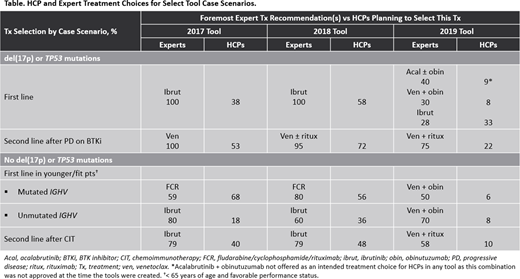
Clear shifts in expert treatment patterns were observed over time (Table). For example, in assessing first-line therapy for patients with CLL with del(17p) or TP53 mutations, the expert panel recommended ibrutinib as first-line therapy regardless of any other characteristic in both the 2017 and 2018 tool iterations; however, in the 2019 tool, experts recommended acalabrutinib plus obinutuzumab, venetoclax plus obinutuzumab, and ibrutinib with similar frequency, dependent upon specific patient characteristics.
Similarly, in younger (< 65 years of age), fitter patients with treatment-naive CLL and no del(17p) or TP53 mutations, IGHV mutation status was important for expert treatment recommendations in the 2017 and 2018 tools, as experts recommended fludarabine/cyclophosphamide/rituximab (FCR) for the majority of cases with IGHV mutations and ibrutinib for those without these mutations in both tool iterations. In the 2019 tool, however, experts had shifted toward the use of venetoclax plus obinutuzumab for 50% of cases with IGHV mutations, with FCR still recommended for 40%; for CLL with unmutated IGHV, experts also shifted to venetoclax plus obinutuzumab (70% of cases).
Substantial variance was observed between expert recommendations and the planned treatment of HCPs for a variety of case scenarios across tool iterations. For example, in the 2019 tool, experts selected venetoclax plus obinutuzumab for 50% of cases of younger patients with treatment-naive CLL with no del(17p) or TP53 mutations but mutated IGHV; HCPs selected this regimen for 6% of these cases. Overall, after reviewing expert recommendations for their cases, 56% of HCPs whose planned treatment differed from the experts indicated that they would change their treatment based on panel recommendations.
Conclusions
Analysis of data from progressive iterations of an online treatment decision support tool suggest evolution in best practices in CLL treatment and differences in how experts and community providers manage patients with CLL. Expert recommendations in the tool changed the intended treatment plan of many HCPs, suggesting that online treatment decision tools providing patient-specific expert guidance may increase implementation of optimal therapeutic decisions for advanced CLL. A full analysis of cases entered into the 2019 tool and comparison with previous tools will be presented.
Awan:Dava Oncology: Consultancy; Kite Pharma: Consultancy; Sunesis: Consultancy; Gilead Sciences: Consultancy; MEI Pharma: Consultancy; Pharmacyclics: Consultancy; Janssen: Consultancy; Abbvie: Consultancy; Astrazeneca: Consultancy; Genentech: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Blueprint medicines: Consultancy. Brown:Gilead, Loxo, Sun, Verastem: Research Funding; Janssen, Teva: Speakers Bureau; Abbvie, Acerta, AstraZeneca, Beigene, Invectys, Juno/Celgene, Kite, Morphosys, Novartis, Octapharma, Pharmacyclics, Sunesis, TG Therapeutics, Verastem: Consultancy. Lamanna:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncternal, Verastem, TG Therapeutics: Other: Institutional research grants, Research Funding; Astra Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; MingSight: Other: Institutional research grants, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Other: Institutional research grants, Research Funding; Octapharma: Research Funding; Loxo: Research Funding; Columbia University Medical Center: Current Employment; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding; Bei-Gene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding. Sharman:Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Acerta: Consultancy, Research Funding; BeiGene: Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding. Zelenetz:MEI Pharma: Research Funding; MorphoSys: Research Funding; Sandoz: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnology: Consultancy; Novartis: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Gilead: Consultancy; Celgene: Research Funding; Roche: Research Funding; Gilead: Research Funding; Genentech/Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal